Drug Launches 2021/22: Is Remote Initiation Support Here to Stay?
COVID has changed the success factors of drug launches. With the benefits of remote initiation being felt by pharma, HCPs, and patients, it will become a necessity beyond the pandemic

- COVID-19 is proving to be a significant challenge for many drug launches; in particular, for specialty medications launching in rather well-served therapeutic areas
- Digital patient support can lower the barrier to prescribing newly launched drugs by remotely assisting treatment initiation and supplying HCPs with real-time insights on tolerability
- With the end of the pandemic being uncertain and a shift to digital channels likely to be permanent, remote initiation turns into a de-risking tactic for any drug launch
In pharma, ‘launch excellence’ is a big deal; about 70% of products that miss expectations at launch continue doing so in subsequent years. Launch preparations start early and need to anticipate the launch environment – but what is that environment going to look like in years to come? How risky will doctors perceive new treatments? Will we see the end of COVID or live with perpetual mutations? Will we see the return of congresses as they once were? And how much face-to-face interactions will sales reps and MSLs have with healthcare professionals in the ‘new normal’?
Drugs Launched During COVID: What We Learned
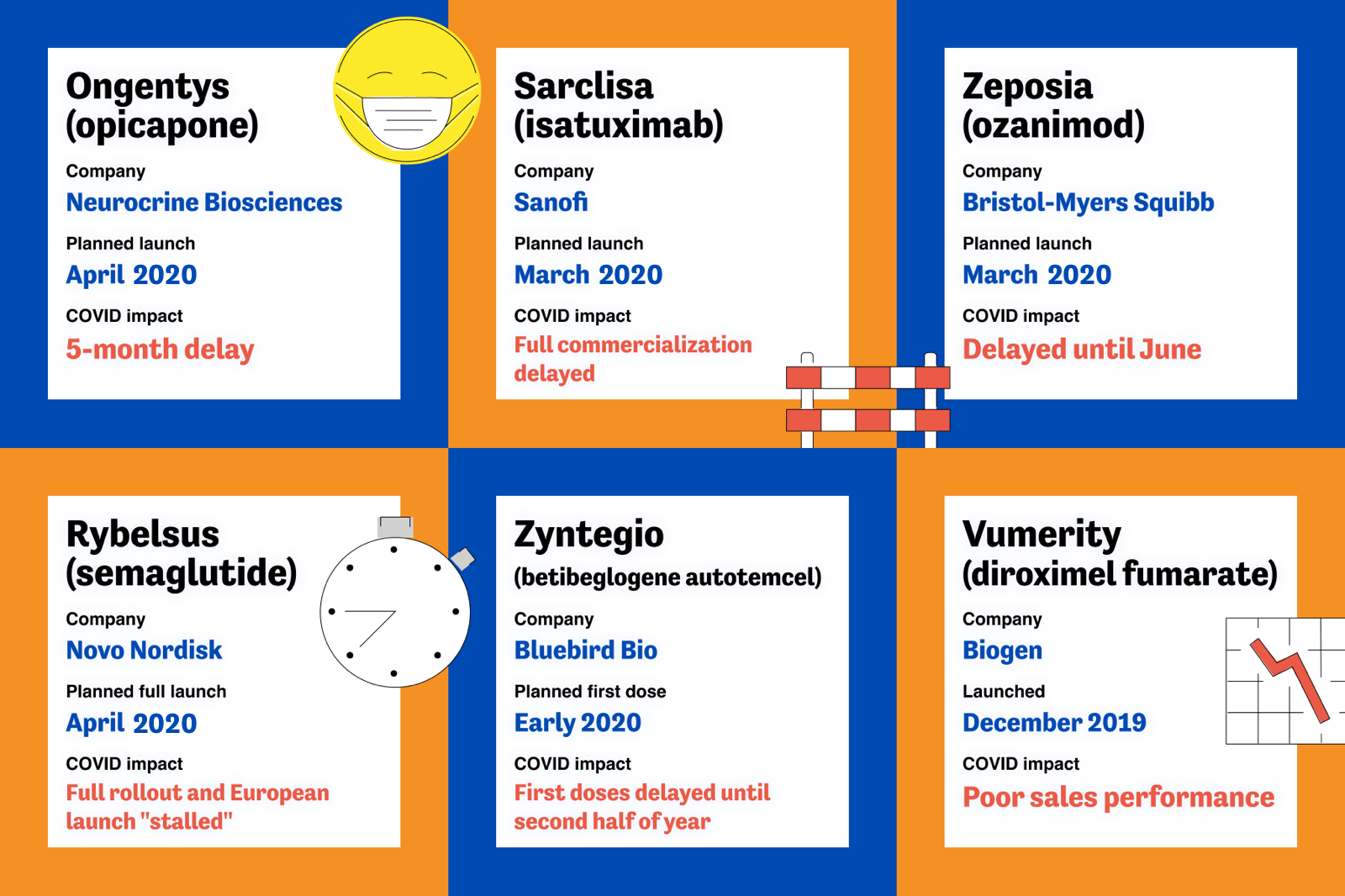
As countries around the globe began introducing restrictions in response to COVID, companies in all industries found themselves needing to adapt. For pharma, drug launches that would typically involve congresses and face-to-face interactions were suddenly thrown into jeopardy. Some launches faced costly delays. Others failed to meet sales expectations, especially those aiming to compete with established treatments, as communication with healthcare professionals was disrupted and information regarding their use in real-world scenarios was limited.
This disruption in communication was less of an issue for drugs that fulfilled an unmet medical need rather than competing with existing treatments. Furthermore, pharma companies that were able to quickly react to the pandemic and pivot to digital technology were able to keep drug launches on track and, in some cases, even exceed expectations. Virtual events were also able to reach far more people than in-person congresses.
While nobody could have anticipated COVID, it seems that preparing for digital distribution and digital care scenarios has proven to be a no-regret strategy.
In regular times, the benefits are wide-reaching. In the event of a sudden change of circumstances, whether local or global, they provide the flexibility for a quick reaction that can help a new product get off to a strong start – which is considered crucial for long-term success. Beyond the launch, though, pharma must also consider its strategy in a new world of patient initiation.
Digital Initiation Support: Why it Matters
Throughout the pandemic, there have been several reasons why doctors have chosen not to prescribe newly launched drugs. Some of the issues that are not directly related to the product, and can therefore be addressed in other ways, include:
- Some products, such as injectables, come with face-to-face initiation or training requirements. In a world of physical distancing, alternatives such as oral drugs are more attractive.
- Without seeing patients regularly, doctors may feel that they lack first-hand insights into real-world tolerability. Consequently, they may view a new product as risky compared to tried-and-tested alternatives.
Pharma can address these issues by taking responsibility beyond the prescription phase and into initiation. The way we support our partners in doing this is by:
- Using MyTherapy to digitally handhold patients during initiation by providing the most critical information and guidance when needed - and presenting it in a patient-friendly language and format.
- Enabling patients to track and remotely share their experience, thus collecting information that can help doctors gain an understanding of a product’s real-world efficacy and tolerability.
By providing these services at product launch, pharma can help close the communication loop that has been so disrupted by COVID and provide patients a more comprehensive initiation. With HCPs now familiar with these tools, their benefits, and their efficiency, they will increasingly become a ‘must-have’ for a new product to compete with existing treatments.
De-risking Your Launch: Prepare for Engaging Patients
While the world is hoping for improved treatment and vaccines to bring an end to the COVID pandemic, issues such as mutations and vaccine uptake make the future an uncertain one. With each passing moment, the healthcare industry is becoming more accustomed to digital tools. The number of digital consultations has increased dramatically and the manner in which pharma communicated with healthcare professionals has likely changed permanently, with face-to-face interactions unlikely to ever return to pre-COVID levels.
Considering what has been learned throughout the pandemic, it seems like a risky strategy to rely on HCPs prescribing newly launched drugs as they did beforehand. Supporting treatment initiation in a remote setting, irrespective of limitations to physical interactions, de-risks a product launch and can become a true differentiator for a new drug.
Digital Initiation Support: We’ve Done it Before
While pharma has a solid understanding of the needs of HCPs, it is often a different story when it comes to patients. Patients are a notoriously heterogeneous group and do not typically fit pharma’s disease-centric thinking and, as a result, have not historically fallen within pharma’s focus.
However, the key success factor for effective initiation support is to meet patients’ needs with a highly accepted and scalable solution. While going digital is a no-brainer, pharma does not typically have the time and capability to start from scratch and iterate until the quality is so good that patients want to engage with the service every day.
This is where we can help. We deploy product-specific initiation support offerings onto MyTherapy, an app already used by millions of patients and that receives over 100 million interactions each month. It is highly scalable and configurable; we are using it for patient support offerings in over 20 markets for products including injectables, orals, and clinic-based treatment.
If you are interested in learning how MyTherapy can provide effective initiation support for your product, get in touch.




