Pharma Digital Medical & Connected Devices: Delivering Value Faster by Setting a Lean MVP
Setting a lean minimum viable product (MVP) in the development of SaMD and digital connected devices can help pharma launch programs faster, while the development of more complex features runs in parallel

- Pharma struggles with the concept of a minimum viable product (MVP), often setting the bar too high for SaMD and digital connected devices
- By launching with a lean MVP, pharma can quickly deliver value to patients, begin collecting real-world data, and drive user adoption and retention
- SaMD features and connected device functionality can be developed in parallel and launched at a later date
Software as a medical device (SaMD) products and digital connected devices are playing an increasingly important role in pharma’s digital ecosystem. However, initiatives are still hampered by long times-to-market, poor user adoption, and programs that fail to provide value to patients. Read how setting a lean minimum viable product (MVP) can help solve these issues.
Digital Medical & Connected Devices: Pharma’s Wayward Approach to the MVP
The concept of a minimum viable product (MVP) is a common one in the world of digital product development. The idea is that a product can be launched when it meets a pre-defined level of functionality and usability, which can then be improved and expanded upon over time. A common analogy is to start with a skateboard and improve with each iteration until you reach a car, rather than starting with four wheels, then adding car components one-by-one. The latter method means the product is not usable until it is a finished car, while the skateboard is already a viable mode of transport.
The concept is somewhat alien to many in pharma, given that a typical pharmaceutical product must be completely finished for it to reach the market. As a result, there are often disagreements about what should constitute the MVP when it comes to digital initiatives.
In particular, we notice that many people in the industry are focused heavily on features rather than value.
When it comes to the development of SaMD programs and digital connected devices, that often means that pharma sets the MVP bar at the point where the specific component that constitutes SaMD or a connected device is completed. In the case of medical devices, this can mean the added delay of attaining regulatory approval.
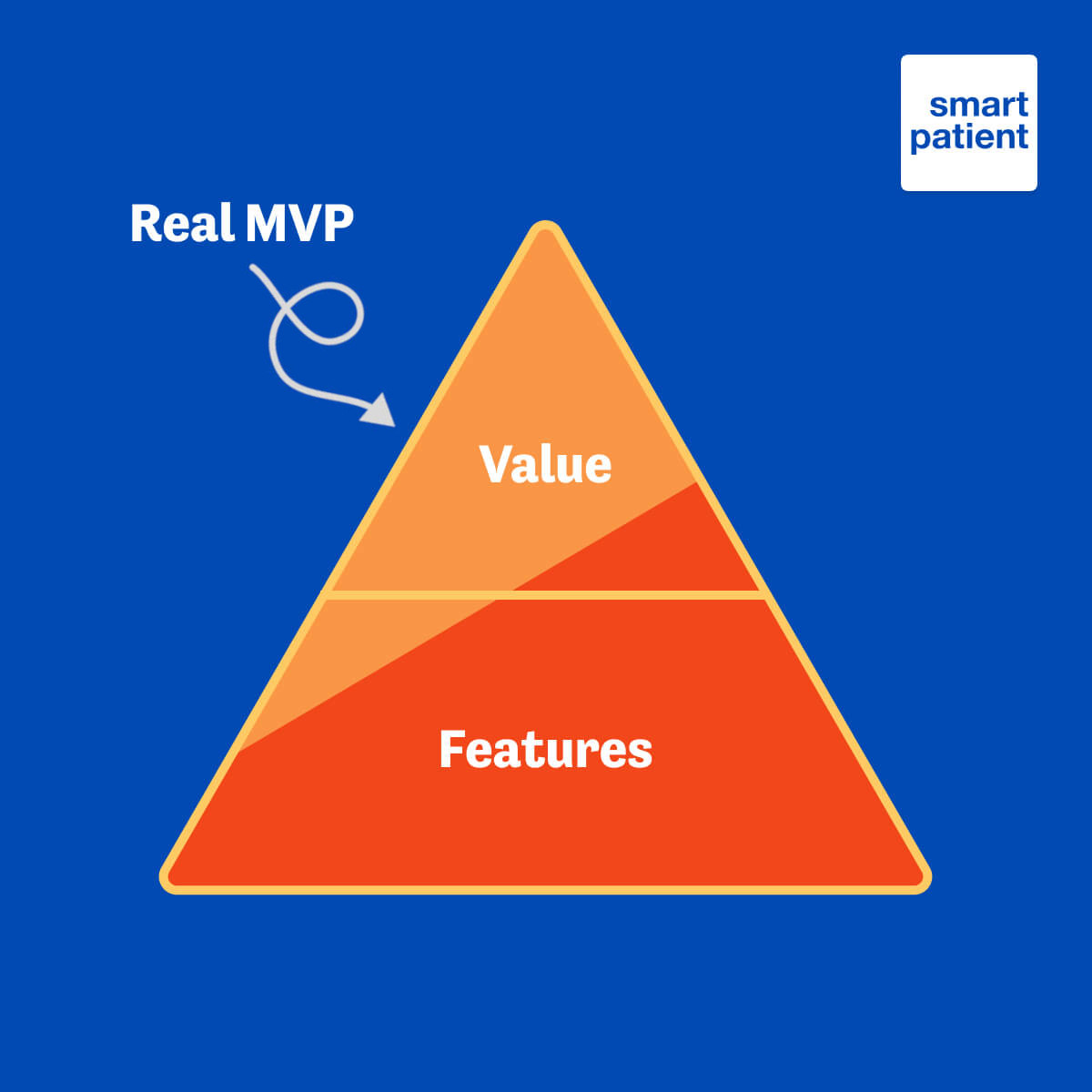
There is a range of problems with this approach. Firstly, it fails to put user needs at the forefront of the development process, which can result in products that achieve some pre-defined criteria but do not provide genuine value to users. Secondly, it is time-consuming and means that while the development is ongoing, no real-world user feedback or insights are being gained. And finally, the combination of the previous two points makes it unnecessarily costly, as the software will still need iterative improvements to deliver value to users.
Furthermore, it can have lasting implications for user adoption, as people who use the product but find little value in it are unlikely to give it another shot – even once improvements have been made.
Despite these issues, it is an approach that continues to be taken by many within the pharma industry. However, there is an alternative approach; by setting a lean MVP and delivering maximum value to users in the early iterations, pharma can begin supporting patients and gaining insights while the development of more complex features runs in parallel.
Start with a Lean MVP & Gain Insights Early
In many cases, SaMD and connected devices launched by pharma comprise of features that, when considered in isolation, do not constitute medical or connected devices. These features can often provide significant value to users by, for example, supporting adherence and persistence or treatment initiation.
Features such as these are often ready to go out-of-the-box and, even if they need adapting for the specific needs of patients living with a certain disease or taking a particular medication, represent the ‘low-handing fruit’ of a digital initiative. A robust digital program containing such features can usually be launched relatively quickly and immediately begin supporting users on a daily basis.
Such an approach means bringing the maximum value to users in the first variation of the product, in keeping with what is considered the best practice when defining the MVP.
This immediate value helps when it comes to user adoption and user retention, meaning pharma also benefits from the data generated by an engaged userbase. Insights from this data can help guide development efforts and ensure that resources are spent on the functionality that will bring increasing value to patients.
This includes guiding the design of the specific feature or features that are considered SaMD or connected devices. Qualitative and quantitative data can help ensure that these complex and time-consuming features achieve the desired aim of supporting patients once they are completed.
Building Digital Medical & Connected Devices on MyTherapy
The lean MVP approach is the one we take when building digital patient services on our MyTherapy platform. With hundreds of iterations, MyTherapy has been developed over many years to meet the needs of millions of people living with chronic diseases.
As a result, it can be quickly adapted to support patients living with a specific disease or taking a particular medication and provide value to users from the beginning.
In parallel, we work alongside our pharma partners to outline the requirements for more complex features, including those that are considered SaMD and connected devices. These features can be added to the program at a later date.
The result is a digital initiative that can be launched faster, delivers value to patients from the start, drives user adoption and retention, generates real-world insights, and is more cost-effective for our partners. Sound interesting? Speak to us about how you can build SaMD programs and connected devices on our MyTherapy platform.